mfloDx™ Diagnostic Platform for AMR
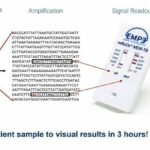
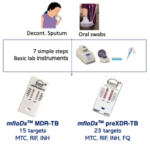
EMPE’s diagnostic platform is inexpensive, easy to use and provides reliable answers about the bacterium and its resistance profile. Our multiplex molecular test indicates the presence of M tuberculosis and its genotypic resistance profile.
Easily scalable
Customisable
Detects multiple diseases
Probes several DNA/RNA mutations
Identifyies microbe and its AMR profile
Only standard lab equipment
No special instruments
Semiskilled personnel
Inexpensive compared to other tests
Sample to result in 3 hours!
- Infection prevention
- Antimicrobial stewardship
- Microbial diagnostics
- Antimicrobial compound/strategy
- Removal antibiotics/bacteria
Microorganisms:
- Bacteria
- Viruses
- Fungi
- Parasites
- Yeasts
Application:
- Human
- Veterinary
- Environmental
- AgriFood
- Other
Development stage:
- Market entry
- Marketed product
- Research
- Development
- Validation
- Company
- Academia
- Institute
- NGO
- Government
Partnering:
- License
- Outsource
- Joint Venture
- Sell
- Co-develop
Funding organisation:
- CARB-X
- FIND
- GARDP
- REPAIR
- OTHER / NA
Infectious disease area:
- RTI
- UTI
- STI
- BSI
- GII
- SSTI
- CNSI
- IAI
- SSI
Geographic origin:
- Eurasia
- North America
- South America
- Africa
- Oceania
N.A.
Nature of organization
Developer of rapid diagnostic tests to detect pathogens and drug resistance. The present focus is on Tuberculosis and drug resistance. EMPE Diagnostics has developed mfloDxTM technology (patents granted) a diagnostic platform combining molecular technologies (padlock probes and rolling circle amplification) and visual biosensors (ultrasensitive lateral flow) to detect the presence of bacteria and antibiotic resistance.
Most of the products available are based on PCR technology but EMPE’s products also use PLP-RCA technology for the detection of multiple nucleotide variations, in multiplex format.
What is going on with AMR?
Stay tuned with remarkable global AMR news and developments!