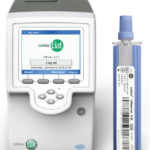
cobas® Liat® System
Next-generation real-time PCR system designed for on-demand STAT testing, enabling use in decentralized locations such as laboratory satellite locations, physican offices and retail pharmacies
Powerful PCR technology with a fast turnaround time, 20 minutes or less, allowing sample to answer while patients wait.
Technology:
Microorganisms:
Application:
Development stage:
- Infection prevention
- Antimicrobial stewardship
- Microbial diagnostics
- Antimicrobial compound/strategy
- Removal antibiotics/bacteria
Microorganisms:
- Bacteria
- Viruses
- Fungi
- Yeasts
- Parasites
Application:
- Human
- Veterinary
- AgriFood
- Environmental
- Other
Development stage:
- Marketed product
- Research
- Development
- Validation
- Market entry
Organization:
Partnering:
Funding organisation:
Infectious disease area:
Geographic origin:
- Company
- Academia
- Institute
- NGO
- Government
Partnering:
- License
- Co-develop
- Outsource
- Joint Venture
- Sell
Funding organisation:
- CARB-X
- FIND
- GARDP
- REPAIR
- OTHER / NA
Infectious disease area:
- BSI
- RTI
- UTI
- STI
- GII
- SSTI
- CNSI
- IAI
- SSI
Geographic origin:
- Eurasia
- North America
- South America
- Africa
- Oceania
N.A.
Molecular, clinical chemistry and immunoassays, tissue, POC, patient self-testing, sequencing, lab automation and IT, and decision support solutions.
C-Reactive Protein assay for diagnosis and management of infection, assessment of inflammation. Elecsys® BRAHMS Procalcitonin (PCT) Electrochemiluminescence immunoassay “ECLIA” for the in vitro quantitative determination of PCT.
What is going on with AMR?
Stay tuned with remarkable global AMR news and developments!