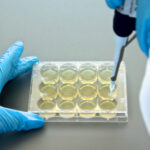
Antimicrobial Susceptibility Testing
Manual, affordable, reliable, sustainable diagnostic devices for microbiology. Development of AST devices with new drugs produced by infectious disease pharmaceutical companies.
Over 200 antibiotics available in our Antimicrobial Susceptibility Testing product ranges.
Customizable devices in terms of antibiotic assortments and concentrations.
- Antimicrobial stewardship
- Microbial diagnostics
- Infection prevention
- Antimicrobial compound/strategy
- Removal antibiotics/bacteria
Microorganisms:
- Bacteria
- Fungi
- Yeasts
- Viruses
- Parasites
Application:
- Human
- Veterinary
- AgriFood
- Environmental
- Other
Development stage:
- Development
- Marketed product
- Research
- Validation
- Market entry
- Company
- Academia
- Institute
- NGO
- Government
Partnering:
- Co-develop
- License
- Outsource
- Joint Venture
- Sell
Funding organisation:
- OTHER / NA
- CARB-X
- FIND
- GARDP
- REPAIR
Infectious disease area:
- UTI
- STI
- BSI
- RTI
- GII
- SSTI
- CNSI
- IAI
- SSI
Geographic origin:
- Eurasia
- North America
- South America
- Africa
- Oceania
N.A.
Since 1983, Liofilchem® produces:
– devices for antimicrobial susceptibility testing such as MTS™ (MIC Test Strip), antibiotic discs, broth micro dilution panels, agar dilution panels, chromogenic culture media for resistance mechanisms detections.
– galleries and biochemical tests for microbial identification.
– ready to use culture media in petri dishes, tubes, bottles, bags and dip-slides.
– dehydrated culture media and growth supplements.
– swabs, contact plates and contact slides for the microbiological monitoring of surfaces.
– bio-indicators for the sterilization process validations and control.
– freeze-dried organisms.
Liofilchem is certified to ISO 9001 for the quality management and to ISO 13485 for the development and production of IVD devices. The Liofilchem products for clinical microbiology are CE marked and comply with the European IVD-D and IVD-R, the US FDA, Health Canada and are registered at the health authorities in many countries around the world.
Logistics, distribution and market services:
Liofilchem’s efficient and flexible distribution centers offer shipping services of RUO devices to voluntary laboratories who wish to try out the new agent against the clinical isolates they encounter.
Following the US FDA clearance or CE IVDR marking, the device becomes IVD labelled and is available for diagnostics usage.
The AST devices are added to our standard catalog and become available for international commercialization.